diabetes blue book
- related: Endocrine, diabetes care in the hospital
- tags: #note
Hyperglycemia inpatient
Any acute illness results in a state of stress with release of hormones (e.g. glucocorticoids) and cytokines, which in turn cause insulin resistance. This increased insulin resistance will exacerbate any underlying defect in carbohydrate metabolism leading to either overt diabetes mellitus or so called “stress hyperglycemia.” Patients admitted with known diabetes have a 3-fold increase in mortality and those admitted with “new-onset” hyperglycemia have an 18 fold increase in mortality.
Many patients with diabetes may be very well controlled as outpatients with one or more oral antidiabetes drugs (OADs) such as metformin, glyburide and sitagliptin. However, these medications require adequate levels of endogenous insulin to be effective. In the setting of acute illness and insulin resistance, it is common for the fasting blood glucose (BG) to exceed 200 mg/dl. When this happens, the patient’s endogenous insulin release is suppressed (so called glucose toxicity) and the BG can potentially exceed 400 mg /dl. BGs of over 500 mg/dl imply either absolute insulin deficiency in type 1 diabetes, or the presence of acute or chronic renal insufficiency or pre-renal azotemia in patients with type 2 diabetes. Pre-renal azotemia is common in the setting of dehydration caused by a hyperglycemic-induced osmotic diuresis. For this reason, all patients with diabetes (regardless of type) as well as those with stress hyperglycemia are best treated with both insulin therapy and appropriate intravenous fluids to control hyperglycemia during hospitalization.
Target Glucose
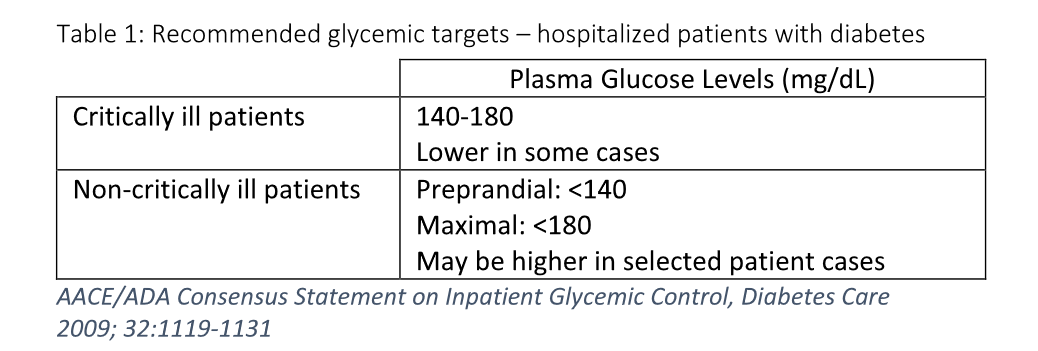
The optimal target range for plasma glucose in hospitalized non-pregnant adult patients is currently being debated. The current recommendations by the American Association of Clinical Endocrinologists and the American Diabetes Association for 2009 are shown in the table below.
Table 1: Recommended glycemic targets – hospitalized patients with diabetes
The American Diabetes Association recommends insulin therapy as the preferred method of glycemic control in the hospital setting in the majority of clinical situations. Physiological insulin replacement therapy will provide the best control and the greatest flexibility during hospitalization.
IV insulin is preferred for the management of hyperglycemia in critical care illness, hyperglycemic emergencies, and selected clinical situations when BG is otherwise difficult to control. Subcutaneous (subQ) basal-bolus regimens are generally recommended for most hospitalized patients. Patients given subQ insulin usually require all three insulin components: 1) basal, 2) nutritional and 3) correctional.
Basics
When hyperglycemia (i.e., blood glucose greater than 180mg/dl) is documented and confirmed, initiation or adjustment of insulin therapy to achieve glycemic control is required.
- Glycemic control for critically ill patients: 140-180 mg/dl - may be lower in select populations.
- Glycemic control for fasting non-critically ill patients: 100-140 mg/dl; for non-fasting patients: <180 mg/dl.
- Hypoglycemia: blood glucose of <70 mg/dl.)
Initial dosing of subQ insulin is based on type of diabetes, weight, and renal function. It is optimally given with one or more of three components:
- Basal – a once-a-day long-acting insulin
- Nutritional – short-acting insulin matching carb exposure
- Correctional – short-acting insulin given for unexpected hyperglycemic episodes with dosing based on a correctional formula ((BG – Target BG) ÷ Insulin Sensitivity Factor).
Diet
Order Consistent Carbohydrate Diet (20-25 cal/kg body weight), unless patient is NPO or receiving non-oral nutrition; may be added as a modifier to other diets (cardiac, clear liquids, etc.) See Table 3 for detailed information.
3 AM FSBS
3am FSBS is needed to assess morning BG and overnight control with basal insulin
- At first initiation of subQ insulin
- When transitioning from IV to subQ insulin
- If patient is admitted for hypoglycemia or experiencing hypoglycemia during admission
- If patient is receiving corticosteroids or evening NPH insulin
- If patient has T1DM, end stage renal disease, or is very thin or elderly
- If blood sugars are fluctuating from high to low during hospitalization
Insulin absorption
- depends on depot breakdown
types
-
Lantus:
- 50% TDD insulin
-
nutritional insulin, lispro: 15 min before eating to 15 min after eating
-
correctional insulin
- (current BS - target) / insulin sensitivity factor
-
ISF: estimated drop in glucose with 1u subQ insulin
-
Daily insulin Dose method - enter patient’s ordered
-
ISF = 1500/TDD
-
TOTAL DAILY DOSE of insulin (Basal + Nutritional) - ISF = 1500/Total Daily Dose
-
Weight Based method takes GFR, type of diabetes, age and BMI into account; Use the LOWEST option (units/kg/d) that applies to a patient
-
OB Weight Based method - enter patient’s trimester
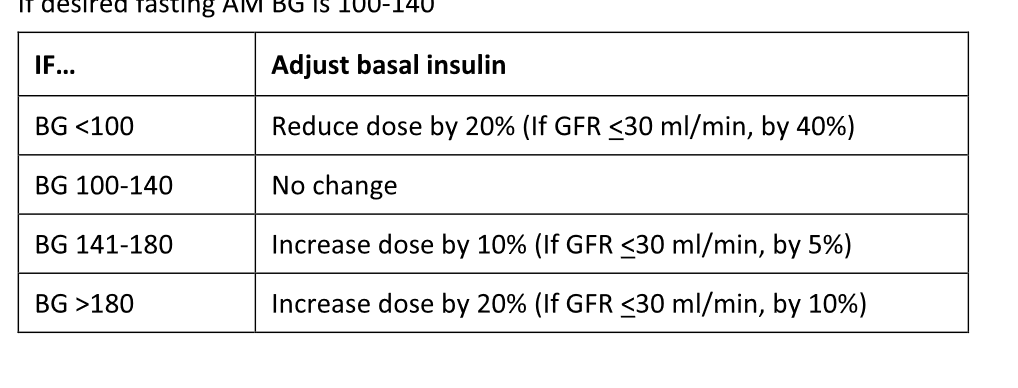
If BG are labile, look for mistiming issues with BG measurement, mealtimes, and insulin administration (e.g., BG checked shortly after a meal, nutritional insulin given when patient did not eat). If so, be conservative with dose changes and discuss mistiming issues with nursing staff.
Lantus/Humalog to Humulin 70/30
Add Lantus and meal time Humalog daily dose to get total daily dose
Give ⅔ of TDD as 70/30 in am 30 minutes before breakfast
Give ⅓ of TDD at 70/30, 30 minutes before last meal of day
Hold nutritional insulin (Humalog, Regular) IF AND ONLY IF patient is NPO or nutrition is interrupted, refused, or very limited. Unless specifically ordered, the current FSBS does not impact the order for nutritional insulin.
Acute and Chronic Kidney Disease
Hypoglycemia is the major concern for CKD patients who are treated with insulin or sulfonylureas. There are several reasons for this propensity to hypoglycemia. First, insulin is metabolized by the kidney. As kidney function is lost, insulin is not cleared normally and both endogenous and exogenous insulin circulate for a much longer period of time. For example, regular insulin may last 6-12 hours instead of typically 4-6 hours with normal renal function. Sulfonylureas are contraindicated in diabetic patients with CKD since they can cause severe hypoglycemia for several days.
Secondly, patients with CKD are prone to hypoglycemia because of the loss of substrate from renal gluconeogenesis. Normally the kidney converts alanine and other amino acids into glucose in the fasting state. With kidney failure this source of calories is lost. If the patient is also eating poorly from uremia or other conditions, their hepatic glycogen may be depleted. In this situation (poor nutrition) there will be limited substrate to mobilize and hypoglycemia from exogenous insulin can be severe and even life-threatening. Most hypoglycemic deaths are felt to be secondary to cardiac arrhythmias.
The dextrose concentration in the dialysis fluid may also affect blood glucose levels (typically increase after peritoneal dialysis and decrease after hemodialysis).
Exercise caution in interpreting hemoglobin A1c levels; they may be falsely low in the presence of hemodialysis, recent blood transfusions, or erythropoietin administration. Insulin dosing at admission
- Initial glargine (Lantus) dose should be 0.1 units/kg/day OR the home dose, WHICHEVER IS LESS.
- For patients on Consistent Carbohydrate Diets, consider using the graduated dosing regimen for patients eating inconsistently
- For patients on continuous TFs or TPN, order lispro (Humalog) every 6 hours for nutritional and correctional insulin; avoid regular insulin
- For correctional insulin component, set Target BG at 140-160mg/dl; ISFs should be consistent with Total Daily Dose of insulin.
Insulin pharmacokinetics are greatly impacted by ESRD
- The impact of dose changes, especially for long-acting insulins, may not be seen for several days. To correct hyperglycemia, make basal insulin dosage changes every 2-3 days.
- Make small changes in insulin doses to correct hyperglycemia and larger changes to correct hypoglycemia.
- Lantus can last 30-36 hours, instead of usual 24 hours.
- Humalog insulin is less impacted by ESRD than any other insulin
- Avoid subQ regular insulin and pre-mixed insulins in ESRD
Endocrinology consults are recommended for ESRD patients with - T1DM
- Peritoneal dialysis
- Steroid treatment
Note: Glipizide (non-sustained release) is the only sulfonylurea that can be used in ESRD, but only as outpatient, not during hospitalization.
Steroid-Induced Hyperglycemia
All inpatients receiving steroids should have point-of-care BG monitoring for the first 24-48 hours to determine if hyperglycemia is present.
When commonly used steroids (e.g., prednisone) are administered once daily in the morning, marked post-prandial hyperglycemia is often seen. When multiple daily doses are administered, or long acting steroids (especially dexamethasone) are used, hyperglycemia is noted throughout the day, including when fasting.
When persistent hyperglycemia (>180mg/dl x2) is documented, treatment is necessary and glycemic goals are the same as for patients with known diabetes. In patients with diabetes, insulin needs may significantly increase when they are receiving oral or intravenous corticosteroids.
Use of correctional insulin alone for patients with steroid-induced hyperglycemia is not recommended since it has been consistently shown to be inferior.
Basal-bolus subQ insulin regimens may be used to effectively control hyperglycemia. However, IV insulin infusions are indicated if BG is consistently >300mg/ml. Modified basal-bolus regimens which contain a lower proportion of basal insulin (30%), and a higher proportion of nutritional insulin (70%), in contrast to the typical 50/50 ratio, are recommended. Lower basal doses also reduce the risk of nighttime or fasting hypoglycemia during steroid tapers.
Proactive reductions during steroid tapers will prevent hypoglycemia. As a rule of thumb, 10-20% reductions in insulin doses may be required for each reduction in steroid dose.
Initial Subcutaneous Insulin Dosing Recommendations
For patients WITHOUT pre-existing diabetes
If BG 140-180 mg/dl, start correctional insulin 5 times daily
If BG >180mg/dl x 2 in 24 hours, begin scheduled insulin 0.4
units/kg/day. (Reduce if GFR <50 ml/min, age >70, BMI <19.)
Divide into 30% basal and 70% nutritional.
For patients WITH pre-existing diabetes
If well-controlled with non-insulin therapies, follow
recommendations for patients WITHOUT pre-existing diabetes
If on insulin or poorly controlled diabetes
T1DM with BMI <25, 0.4 units/kg/day
T1DM/T2DM with BMI 25-30, 0.5 units/kg/day
T2DM with BMI >30, 0.6 units/kg/day
Reduce above daily insulin dose by 10-20% in patients at
high risk for hypoglycemia
Divide daily dose: 30% basal, 70% nutritional
perioperative
Peri-Operative Patients
Day before procedure
- Usual doses of daytime insulin until patient becomes NPO
- Usual dose of basal insulin at night UNLESS patient has been experiencing night-time or early-morning hypoglycemia in the previous 2 weeks, then, give 50-80% of usual dose
- If patient is using a home insulin pump during hospitalization, consult Endocrinology for recommendations
- Continue correctional insulin, administered every 4 hours (Humalog) or every 6 hours (regular insulin) The day of procedure
- No oral meds or pre-mixed insulin
- 50% of usual basal dose if taken in morning
- No nutritional/mealtime insulin
- Continue BG monitoring and correctional insulin as needed
- If procedure lasts > 3 hours, or patient is being closely monitored in ICU, continuous IV insulin infusion is preferred. Post procedure
- Continue BG monitoring and correctional insulin as needed
- Continue basal insulin as scheduled
- Add nutritional/mealtime insulin when feeding resumes.
TPN
Total Parenteral Nutrition (TPN): Use Subcutaneous Insulin Order Set
• Check FSBS every 6 hours.
• Order correctional regular insulin every 6 hours using weight-based
ISF and target 140mg/dl.
• Patients with pre-existing diabetes will need basal insulin.
• Nutritional insulin component may be added to TPN or given on a
scheduled basis as regular insulin every 6 hours; adjust as needed.
Nutritional insulin up to 60% of the total daily dose may be required.
• Note: In patients with End Stage Renal Disease, lispro (Humalog) is
preferred, and may be given every 6 hours.
• The sole use of long-acting insulin is discouraged. Sudden
discontinuation or interruption of feedings can translate into severe
hypoglycemia.
Enteral Nutrition: Use Subcutaneous Insulin Order Set
• Continuous Tube Feedings - Managed like TPN
Starting dose
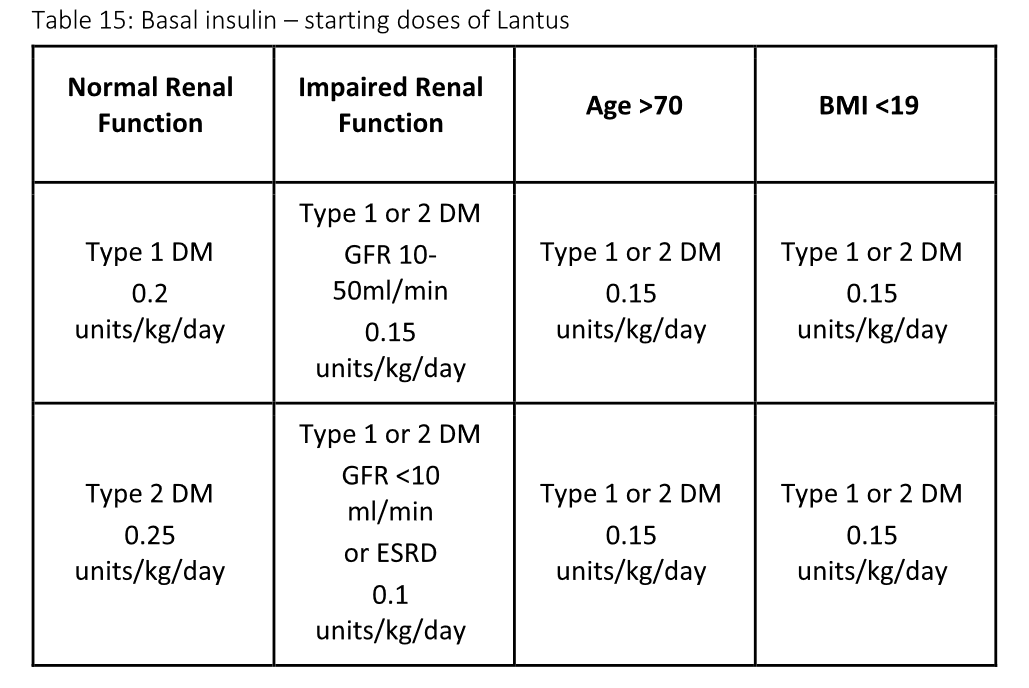
Calculate Corrective (Supplemental) Insulin: a. Weight Based (Wake One order set is based on Type of DM and weight): 3400 ÷ weight (kg) = Insulin Sensitivity Factor (ISF) b. Based on TDD If Known: 1700 ÷ TDD = Insulin Sensitivity Factor (ISF)
For dosing based on TDD: each class of non-insulin anti-diabetic agent (e.g. sulfonylurea, metformin) as equivalent to 20-30 units of insulin. Proportional decreases in insulin equivalency when dosed less than maximally.
Transitioning from IV to Subcutaneous Insulin: Must give first subcutaneous basal insulin dose at least 2 hours before stopping IV insulin!
- Estimate hourly insulin rate in units per hour over past 4 hours.
- Multiply this rate by 24 hours to estimate total IV insulin need per 24 hours.
- Use 80% of this 24 hour total as initial Basal Insulin dose per 24 hours.
- If patient eating, add Prandial Insulin (eg. Lispro) starting at 0.1 unit/kg body weight SQ TID pc each meal. (Hold dose if unable to eat).
- Order corrective insulin based on weight or TDD